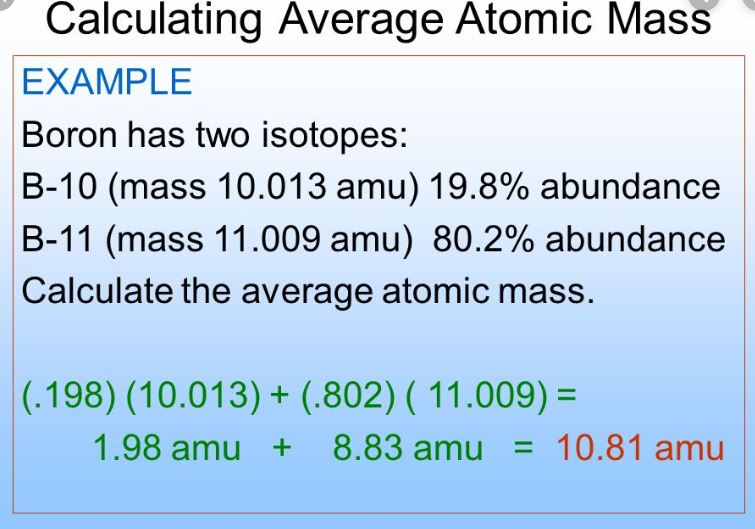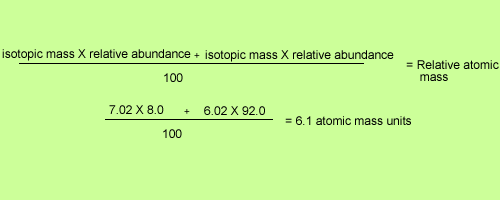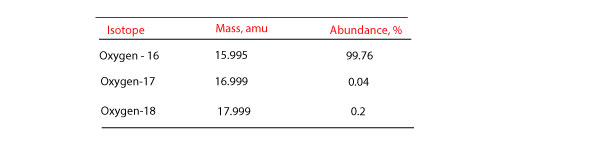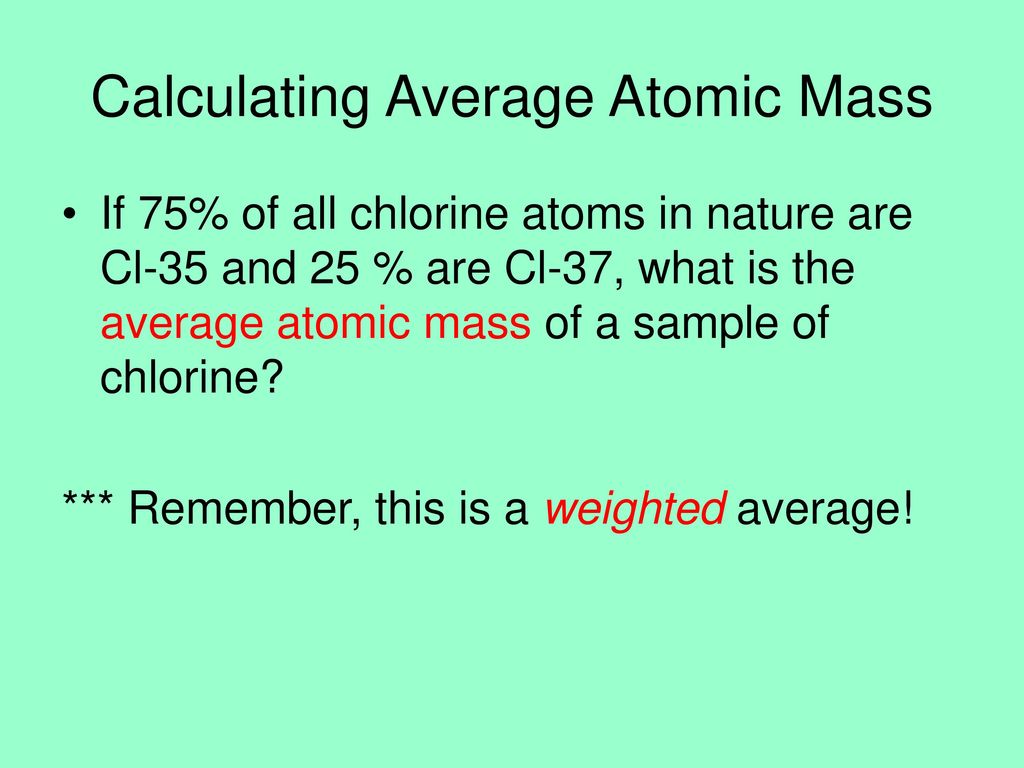
Average Atomic Mass In nature, most elements are a mixture of different isotopes The mass of a sample of an element is a weighted average of all the isotopes. - ppt download

Atomic masses L.O.: Define the terms relative isotopic mass and relative atomic mass, based on the 12C scale; Calculate the relative atomic mass of. - ppt download

Calculate Relative Atomic Mass (1.3.2) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams
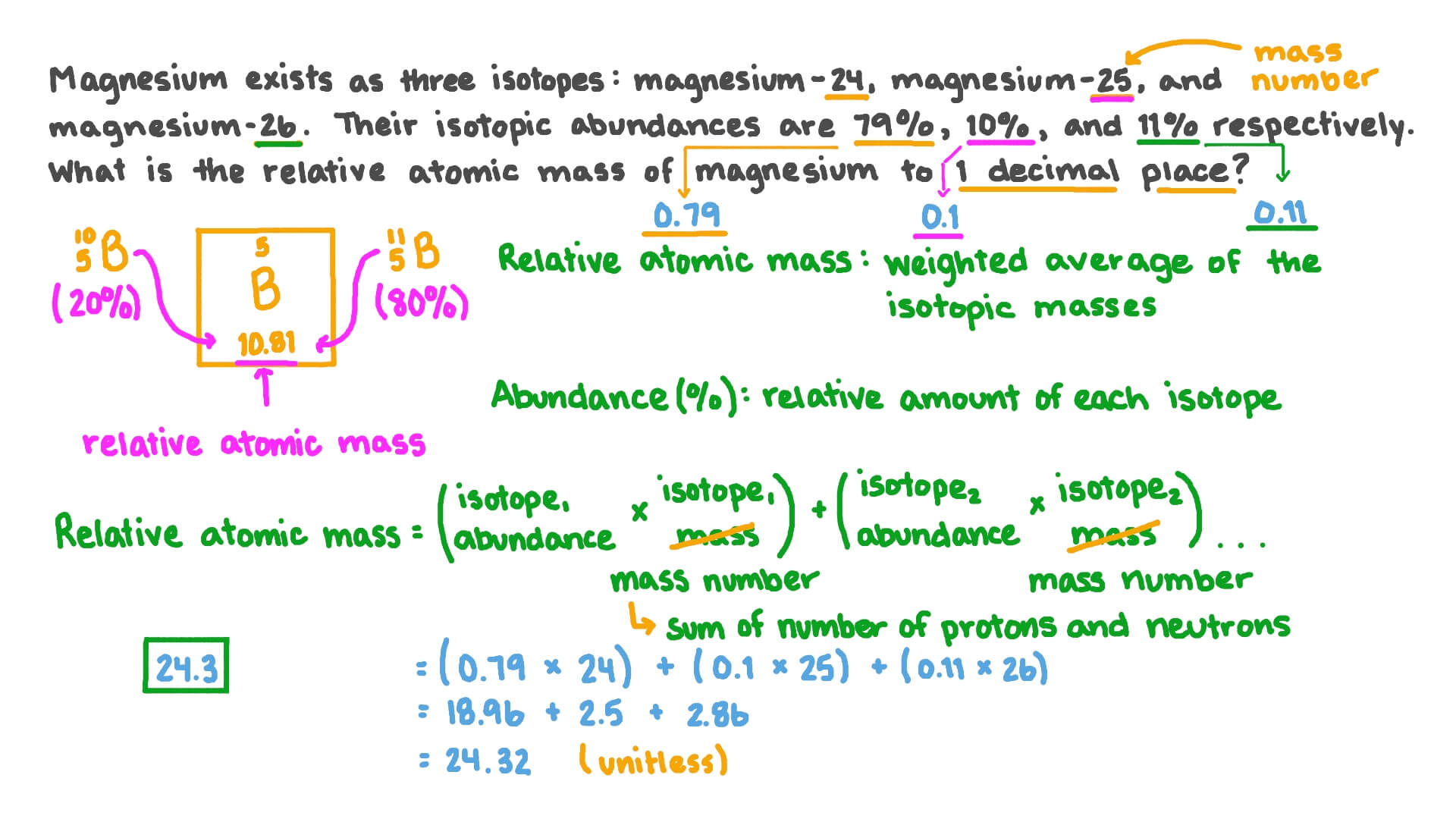
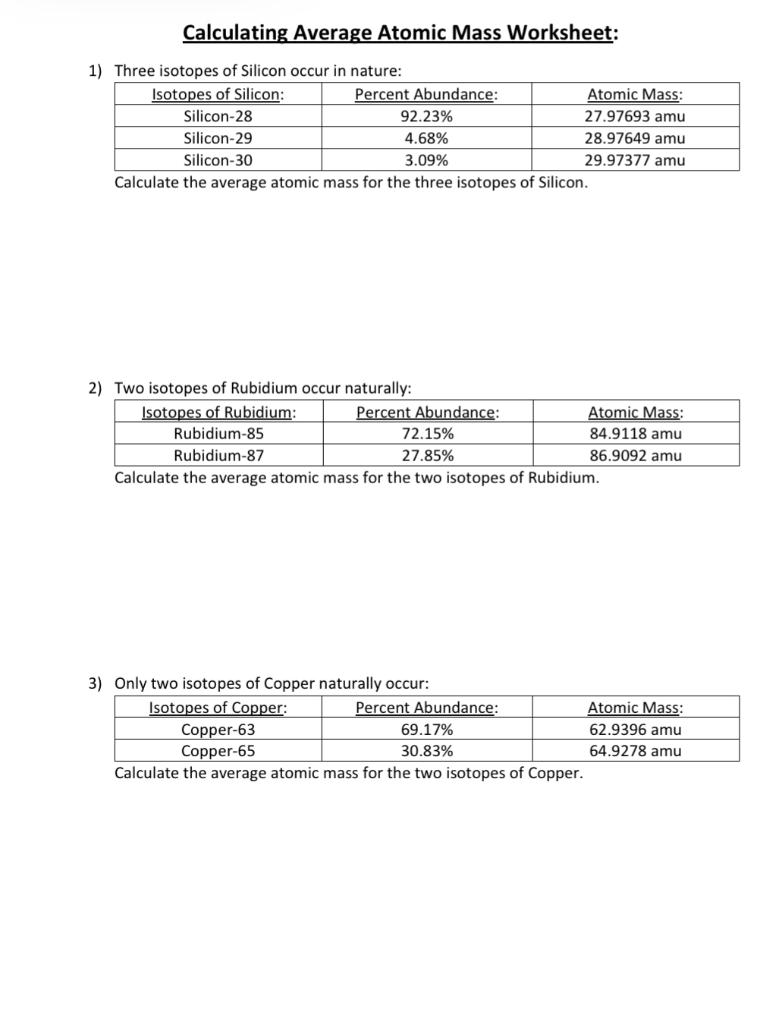
The table below show the relative atomic masses and the percentage abundance of the isotopes L1 and - Tutorke