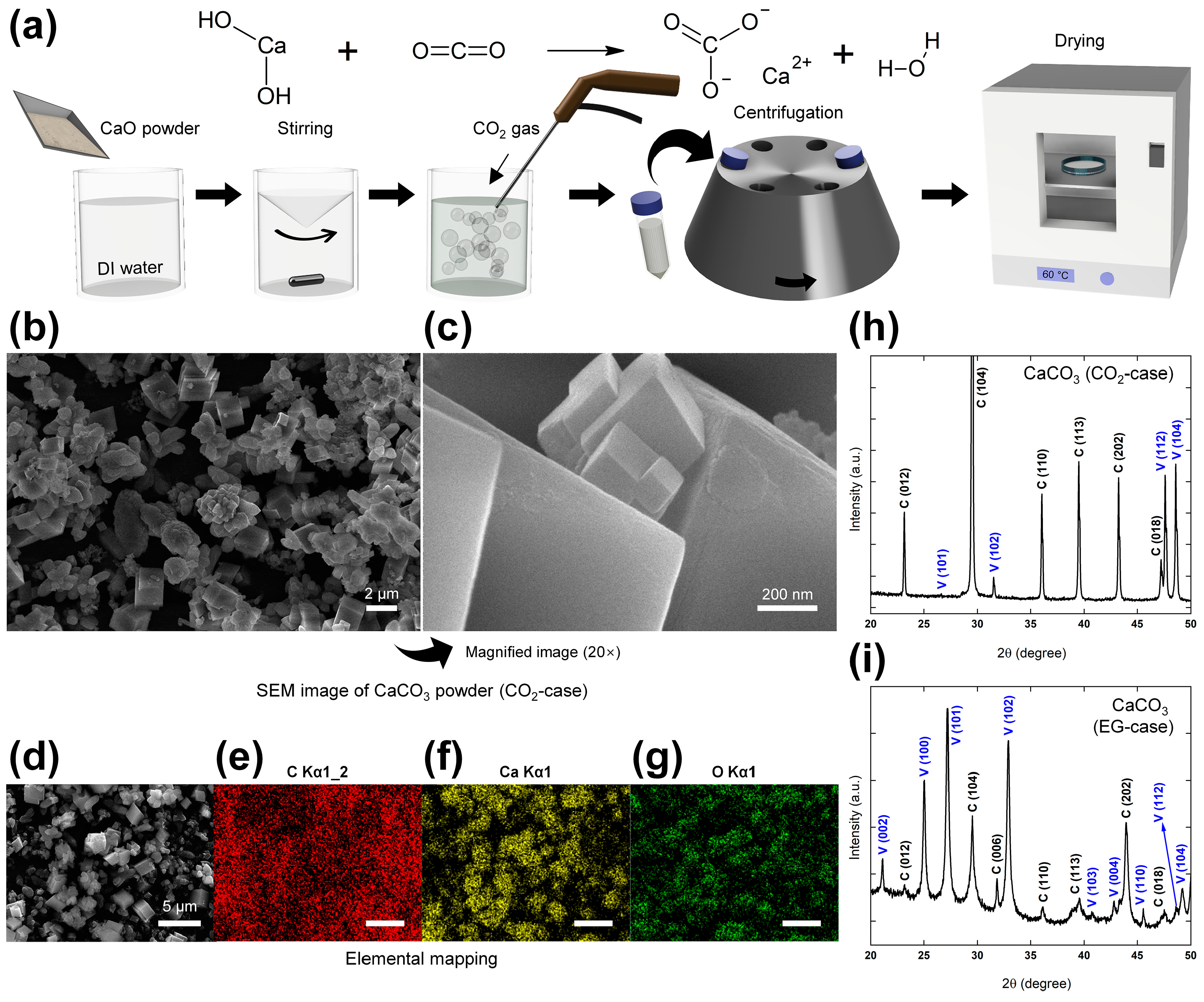
Micromachines | Free Full-Text | A Robust Triboelectric Impact Sensor with Carbon Dioxide Precursor-Based Calcium Carbonate Layer for Slap Match Application
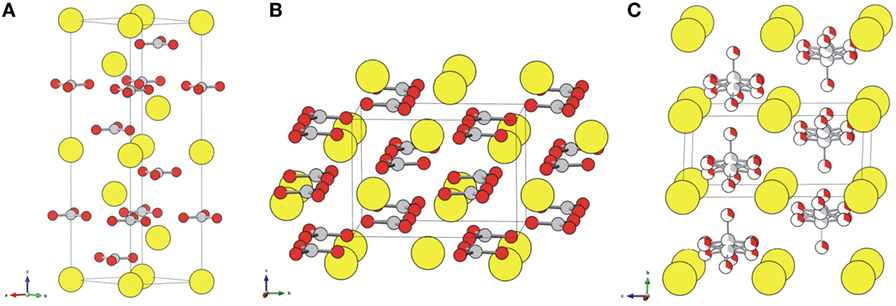
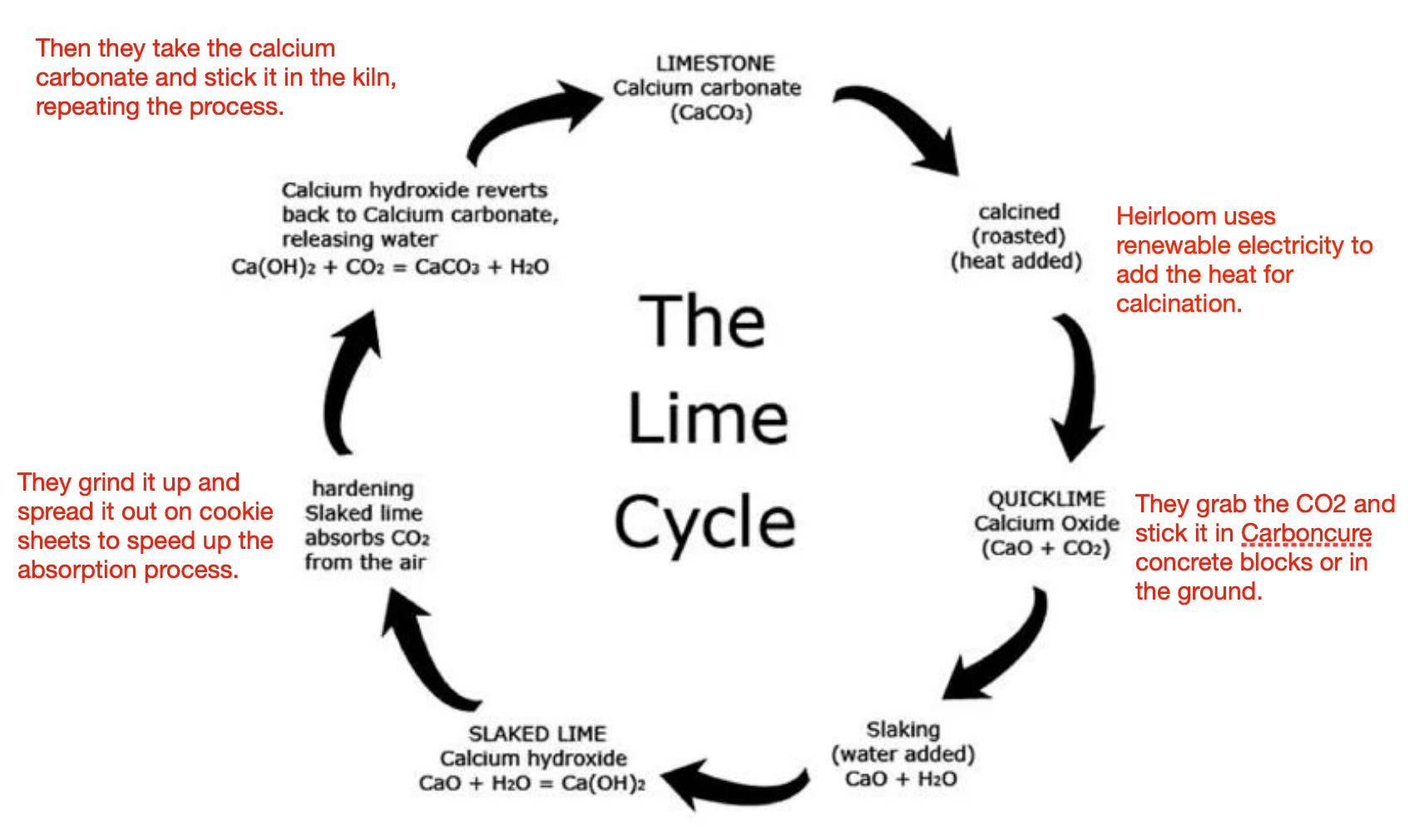
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism

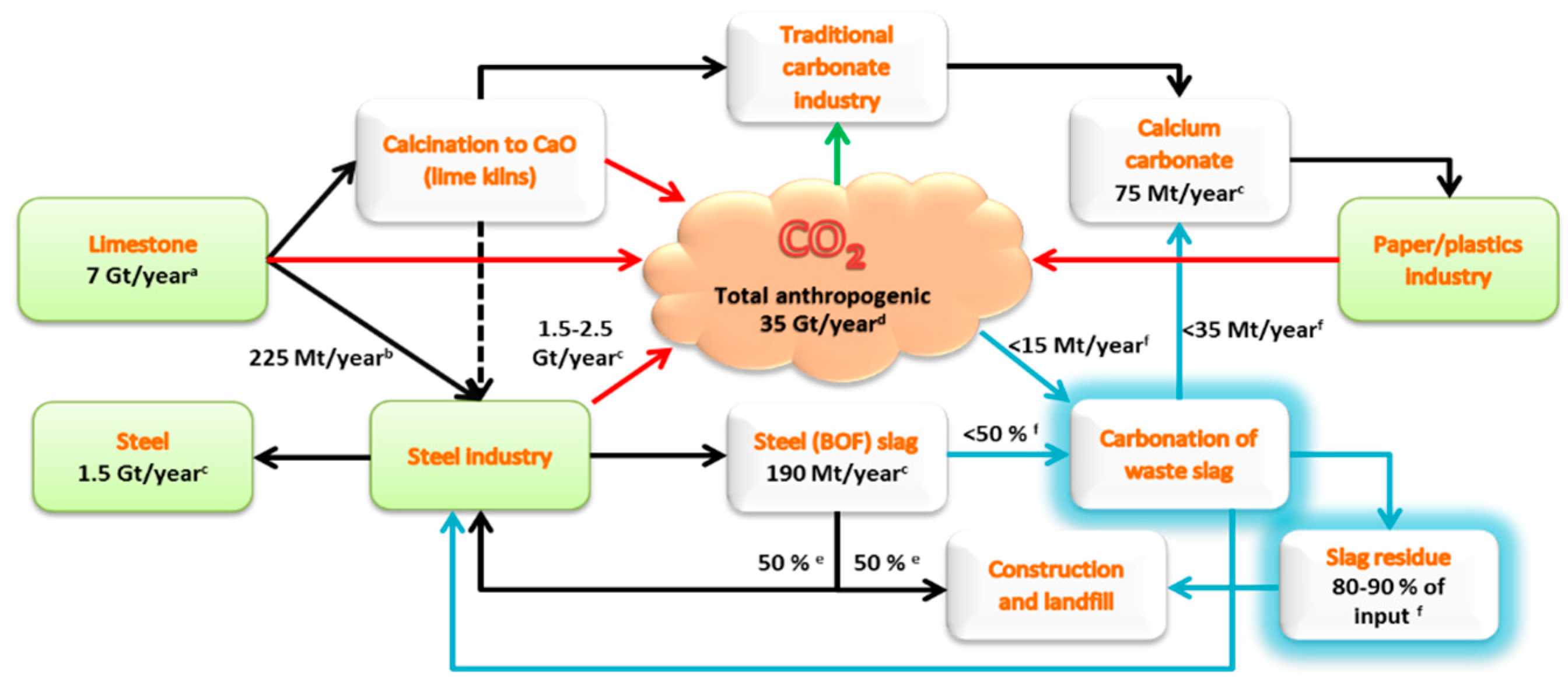
Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag - ScienceDirect

Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram
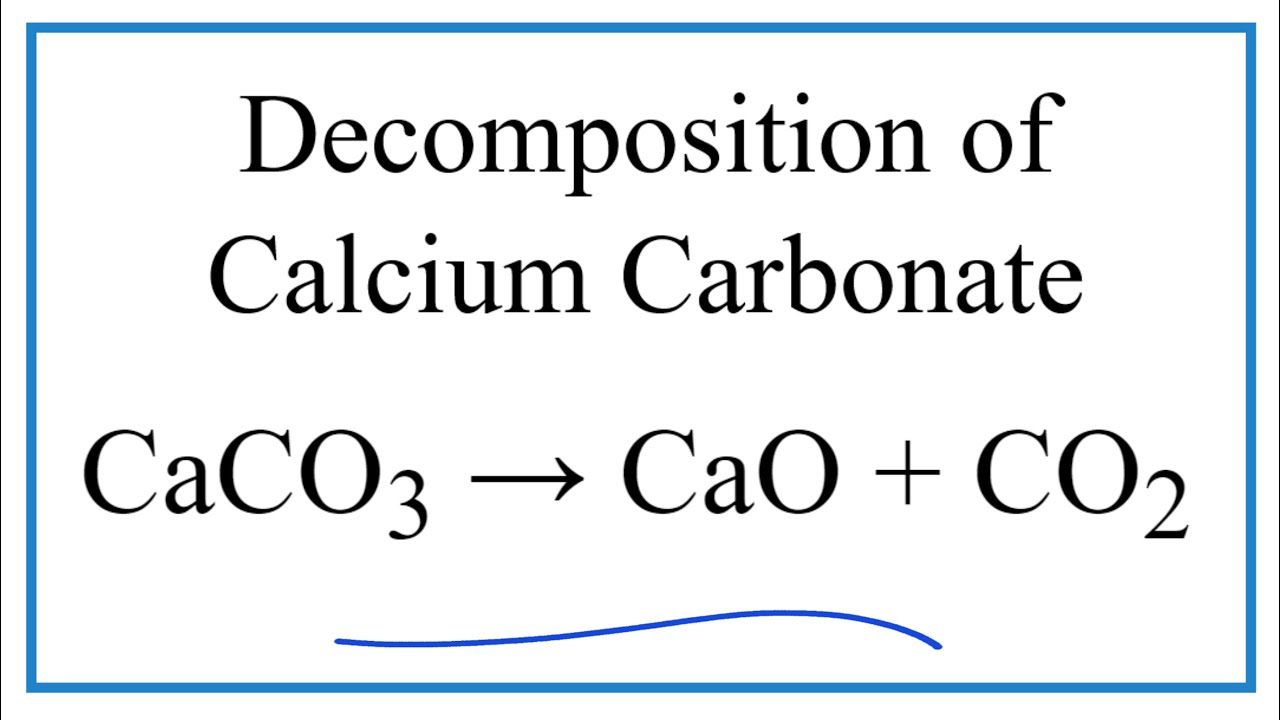
Heating Limestone (CaCO3) produces calcium oxide and carbon dioxide. The equation for this is: CaCO3(s) —> CaO(s) + CO2(g) If we heated up 300g of calcium carbonate. What mass of calcium oxide

Co-utilisation of CO2 and Calcium Silicate-rich Slags for Precipitated Calcium Carbonate Production (Part II) | Semantic Scholar

One method of determining the proportion of calcium carbonate in a coral is to dissolve a known mass of the coral in excess acid and measure the volume of carbon dioxide formed.
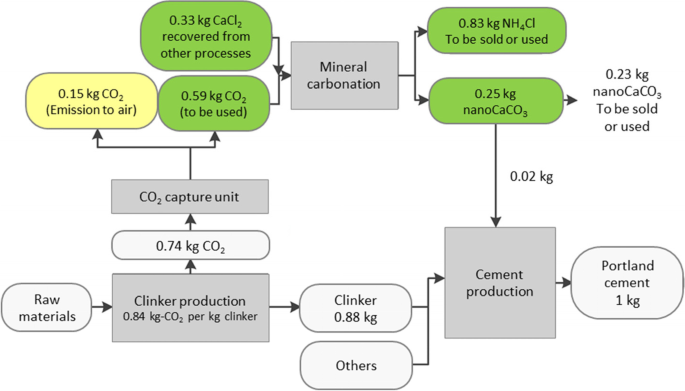




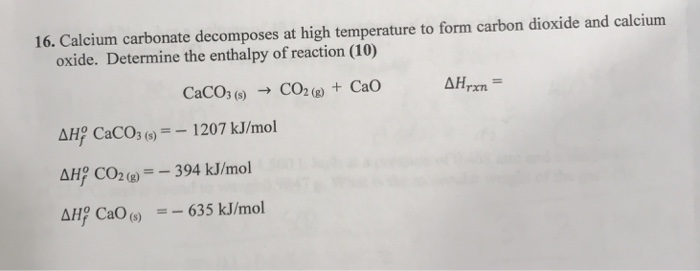
![PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70ca22dce93c5e0c4e267a02ccc4e41951e44132/2-Figure1-1.png)