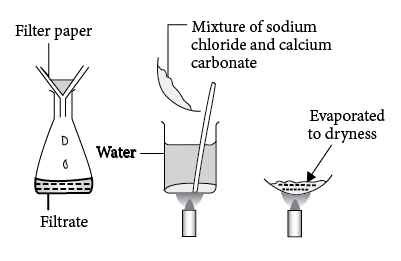
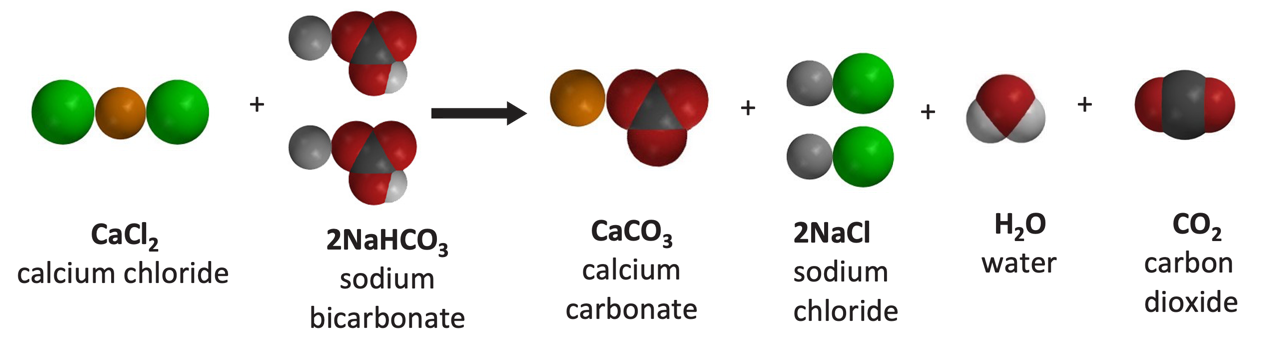
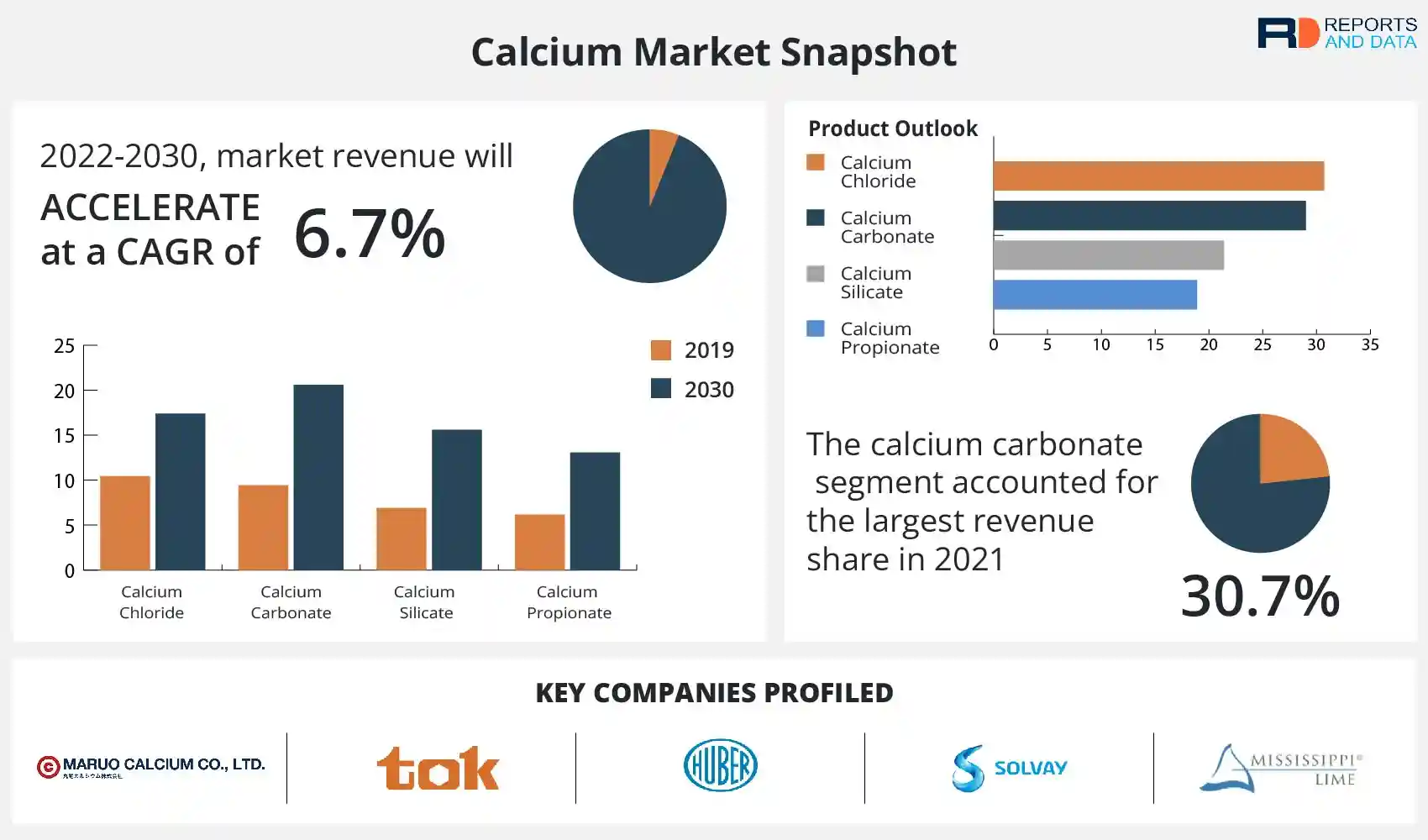
SEM image showing the effects of the use of different calcium salts on... | Download Scientific Diagram

Coatings | Free Full-Text | Evaluating the Protective Effects of Calcium Carbonate Coating on Sandstone Cultural Heritage

What happens when CaCl2 reacts with Na2CO3 | CaCl2 + Na2CO3 | Calcium chloride + Sodium carbonate - YouTube
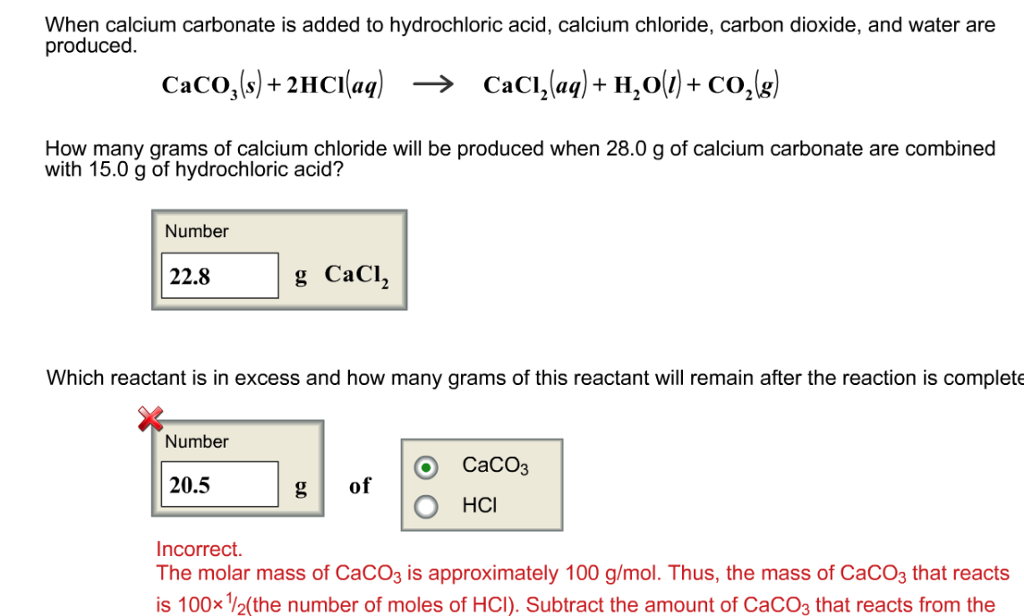
Q. Calcium carbonate reacts with aqueous HCL to give Calcium chloride and carbon dioxide ,according to the reaction CaCo3(s)+2HCl(aq) →CaCl2(aq)+CO2(g)+H2O(l) What mass of calcium carbonate is required to react completely with 25
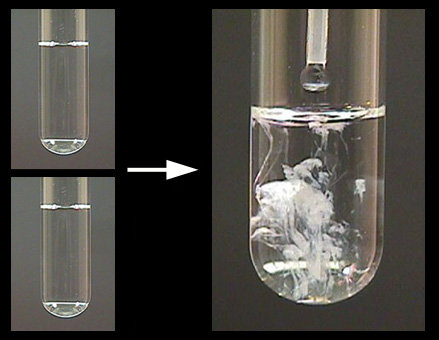
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic

What precipitate will form when aqueous solutions of sodium carbonate calcium Na_2CO_3 and calcium chloride CaCl_2 are mixed? | Socratic